NY Woman Claims ParaGard IUD Broke In Her Uterus
NY Woman Claims ParaGard IUD Broke In Her Uterus
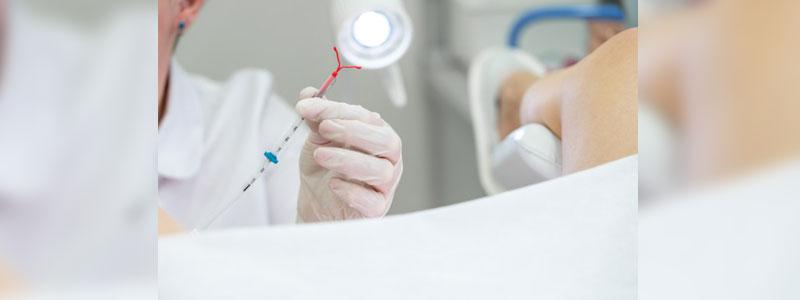
Introduction
Last Monday, a New York woman, filed a lawsuit against Teva Pharmaceuticals USA Inc. and the Cooper Companies Inc. in the state's federal court, alleging that the ParaGard intrauterine device (IUD) broke off in her uterus during removal.
According to the lawsuit, the plaintiff had the device implanted in June 2017. In September, the same year, she went to have it removed after an ultrasound showed it moved out of place. Her physician tried to remove the device following Teva's instructions, but a part of the device broke off, resulting in surgery to have the remaining part of the device removed. Johnson filed the suit claiming that she suffered a range of injuries, including loss of reproductive health, pain, suffering, mental anguish, and loss of enjoyment of life, on top of the medical bills.
The lawsuit also claims that Teva amplified the benefits of the device and knew or should have known about its defects based on trials, third-party studies, and consumer experience and complaints. The lawsuit also names the Cooper Companies and a subsidiary as defendants, as they purchased the rights to the ParaGard in September 2017.
The complaint indicated that more than 1,600 cases of the ParaGard breaking were reported to the U.S. Food and Drug Administration (FDA), out of those more than 700 were deemed serious.
The plaintiff is seeking undefined damages for 11 different counts, including negligence, common law fraud, breach of express and implied warranty, design and manufacturing defects, failure to warn, and violations of consumer protection laws.
Several lawsuits have been filed against the birth control device involving similar allegations of problems during removal surgery. The lawsuits are pending in their individual courts, and an MDL is yet to be formed.
Latest News
Texas Trial to Decide J&J’s $10B Talcum Powder Settlement
A high-stakes trial in Texas will determine whether Johnson & Johnson (J&J) can resolve tens of thousands of talcum powder cancer lawsuits through a…




