Purdue Pharma Reaches $116M Settlement With British Columbia
Purdue Pharma Reaches $116M Settlement With British Columbia
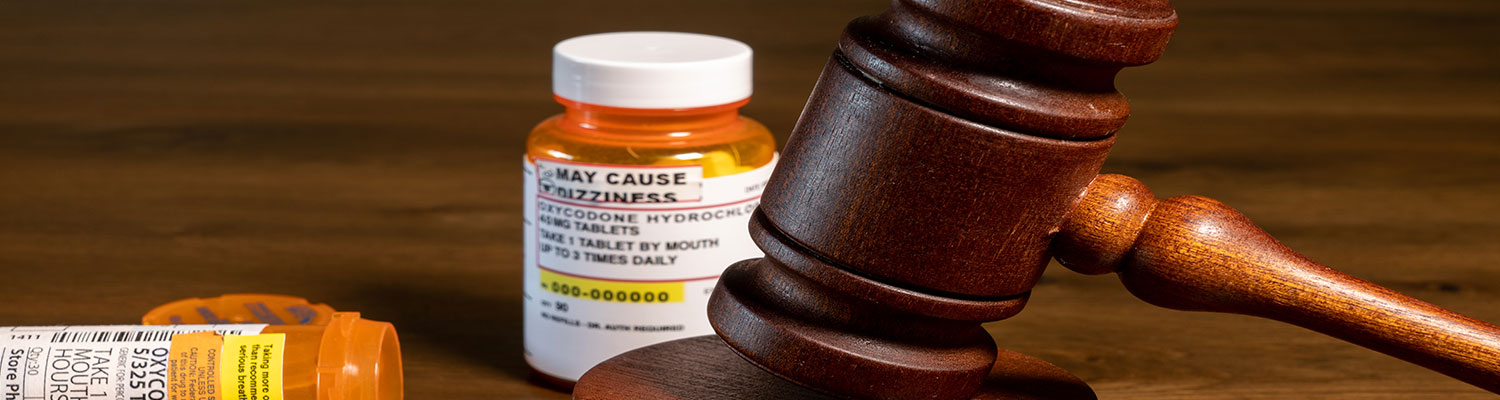
Introduction
British Columbia, Canada, has reached a settlement with OxyContin manufacturer Purdue Pharma Canada for $150 million in medical expenses associated with the opioid crisis.
Purdue is one of the more than 40 medication manufacturers and distributors identified as defendants in a proposed class-action lawsuit brought by British Columbia on behalf of all provincial and federal governments of Canada in 2018. Health care expenses related to misconduct by opioid producers, distributors, and their consultants are the focus of the complaint.
The proposed settlement with Purdue Canada, which totals $150 million in financial rewards and other advantages, including access to information and records relevant to the claim, has been discussed with the federal, provincial, and territory governments.
It represents the biggest public health litigation settlement in Canadian history. According to the Purdue Pharma Canada spokesperson, the organization has agreed to pay the fine and provide the prosecution access to the documents it has asked in exchange for being freed from any future and prior claims and obligations from the government over opioid use.
In Canada, there were 29,052 apparent opioid-related fatalities between January 2016 and December 2021, with 7,560 of these deaths occurring in 2021, according to official statistics. The number of these deaths surged by 96% in the first year of the COVID-19 epidemic.
No amount of money can bring back the deceased, according to the Attorney General of British Columbia, but we are dedicated to holding businesses and other individuals responsible for any misconduct in the production and distribution of opioids.
Purdue Pharma Canada or any of its affiliated parties have not admitted any wrongdoing or culpability as part of the settlement, the company stated in a statement sent by email.
Latest News
Texas Trial to Decide J&J’s $10B Talcum Powder Settlement
A high-stakes trial in Texas will determine whether Johnson & Johnson (J&J) can resolve tens of thousands of talcum powder cancer lawsuits through a…




