Washington To Get $18M From Mallinckrodt Over Opioid Crisis
Washington To Get $18M From Mallinckrodt Over Opioid Crisis
Introduction
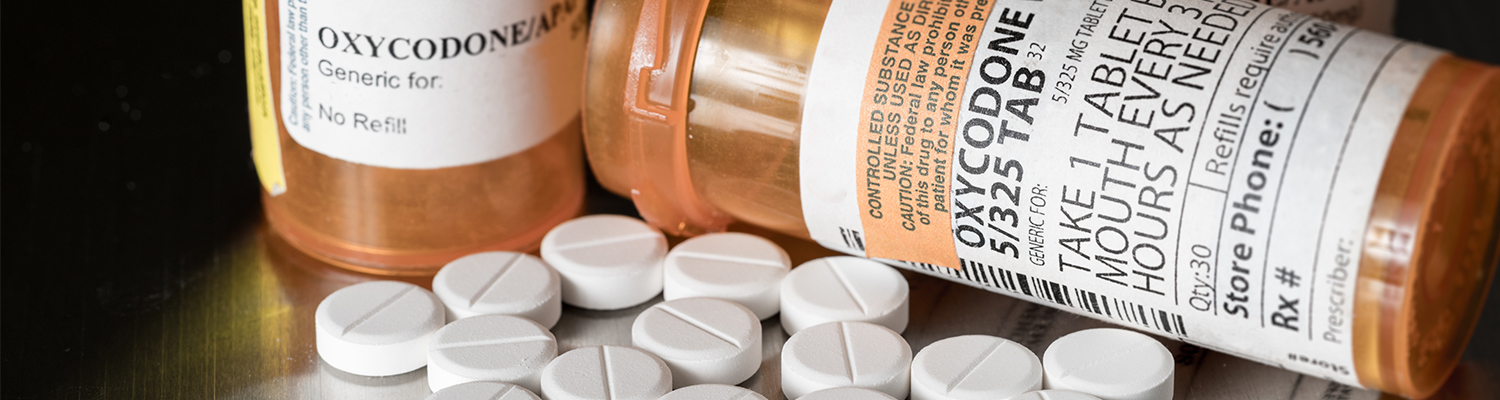
The drug manufacturer Mallinckrodt which has been announced to be bankrupt will have to pay an $18 million settlement to Washington over its role in fueling the opioid crisis in the state.
Washington's attorney general said that the company's share in the opioid settlement might exceed $27 million if it considers paying the amount for a longer period of time instead of disbursing the lump sum amount. The company needs to decide the method of payment within 18 months.
Mallinckrodt will also pay $514,702 for underpayment of Medicaid rebates over a drug it produced called “Acthar” as part of the bankruptcy.
These payments will support the state to increase prevention efforts and help the affected people to deal with the crisis. The AG stated that the companies responsible for fueling the opioid crisis must help the state address the crisis. He even added that the attorneys will continue the fight against the epidemic to deliver as many resources into the communities.
The company faces several investigations and lawsuits from multiple state attorneys general and filed for bankruptcy in October 2020, citing the huge amount of payments that total up to $1.2 to $1.6 billion to resolve the liabilities.
Mallinckrodt manufactures a generic version of oxycodone and is one of the largest pharmaceutical manufacturers in the U.S., with its corporate headquarters located in Ireland. As per the database provided by U.S. Drug Enforcement Agency, one of the subsidiary companies of Mallinckrodt distributed 28.9 billion oxycodone pills from 2006 to 2012 across the nation accounting for up to 80 pills for each person in the U.S.
Latest News
Texas Trial to Decide J&J’s $10B Talcum Powder Settlement
A high-stakes trial in Texas will determine whether Johnson & Johnson (J&J) can resolve tens of thousands of talcum powder cancer lawsuits through a…




