What Happened In The MassTorts World Last Week? 2020-Jun-22
Court Dismisses Taxotere Lawsuits Filed After December 2015

Taxotere lawsuits' presiding Judge Jane Triche Milazzo passed a defense motion to dismiss the cases filed after December 2015, when new warning labels were added about the risk of permanent hair loss associated with the chemotherapy drug.
A court order over the dismissal was issued on May 27 by Judge Milazzo. The motion included nearly 200 cases that sued Sanofi-Aventis, manufacturer of chemotherapy drug, Taxotere, administered for the treatment of breast cancer, or other forms of cancer. The lawsuits allege that the drug caused permanent alopecia, also known as permanent hair loss.
The motion rooted in the fact that in December 2015, the Taxotere label was updated to specifically warn about the risk of permanent alopecia, asking the court to grant summary judgment against these hundreds of plaintiffs as they were treated after the label change. The defendants alternatively argued that their claims were preempted.
Taxotere, also known as docetaxel, is a chemotherapy drug that doctors prescribe to treat several different cancers, mostly breast cancer. While the drug is effective at treating breast cancer, it is associated with several side effects, ranging from common skin reactions to very rare instances of leukemia.
Since Taxotere is a strong chemotherapy drug, its side effects tend to be more extreme than drugs that treat less serious issues such as high cholesterol or blood pressure. Doctors may lower the dose or prescribe drugs that reduce the risk of allergic reactions to deal with these types of side effects.
The drug carries a black box warning that includes five complications that can be severe or fatal: toxic death, low blood cell counts, liver toxicity, fluid retention, and hypersensitivity reactions.
Taxotere can occur during treatment or shortly after. Doctors check liver, kidney, and bone marrow function to make sure a patient can tolerate the chemotherapy drug and that any acute reactions can be treated.
Low white blood cell counts, also called neutropenia, can occur in people who take Taxotere. A more serious version accompanied by fever is called febrile neutropenia. Sometimes, it can be serious enough to cause an infection that requires hospitalization.
Neutropenia is a common side effect of most chemotherapy drugs. Usually, white blood cell counts drop around 10 days to 14 days after patients first get chemotherapy.
The first bellwether trial, held in September 2019, favored the drugmaker, over which the plaintiff is currently appealing. Plans for three other trials have been put on hold due to the current COVID-19 pandemic. Also, according to a case management order issued on May 26, the addition of a fifth bellwether trial along with four more claims to the pool was announced by Judge Milazzo.
Cosmetic Giants Withdraw From Using Talc In Products

Three biggest cosmetic manufacturers Chanel, Revlon, and L'Oreal, have joined Johnson & Johnson's (J&J) move of removing talcum powder from their products due to growing concerns over the mounting cancer lawsuits.
Chanel removed talc from a loose face powder and dropped a talc body powder, Revlon Inc removed talc from its body products, and L'Oreal SA is searching for alternatives for the minerals, as reported by the companies. Chanel, which will continue to use talc in other products including pressed powder, blush, and eye shadow, said all the talc it uses is "selected according to strict purity criteria, fully complies with current global regulations, and is safe under standard conditions of cosmetic use."
Last month, J&J decided to stop selling its talcum products in the U.S. and Canada due to a decline in consumer demand and claiming misinformation about the safety of the products.
The manufacturers and distributors of talcum powder are involved in several lawsuits, each alleging that the asbestos present in the talc causes different forms of cancer. Asbestos is a compound of six naturally occurring silicate minerals that are resistant to heat, corrosion, and some chemicals. Asbestos is harmful to our health. According to the International Agency on Research for cancer, all types of asbestos are listed as "carcinogenic to humans." A carcinogen is defined as a substance that tends to cause cancer. It is considered a carcinogen because of the effects asbestos fibers can have on the human lungs.
Since 2013, many cancer lawsuits have been filed against Johnson & Johnson (J&J). In 2017 Allegations started that asbestos contamination caused plaintiffs’ cancers. Other makers of talc powders also face lawsuits, including Revlon, Chanel, and Avon, securities filings, and court records show.
Talc powder is used in many cosmetics and personal care products to absorb moisture in products, prevent caking, and increase smoothness.
In an analysis conducted last year by the U.S. Food and Drug Administration, asbestos was actively found in 9 out of 52 talc-containing cosmetic products, all of which were recalled voluntarily. The FDA is analyzing 50 more samples this year and is considering establishing an asbestos testing standard.
The talcum lawsuits are consolidated under multidistrict litigation (MDL) no. 2738, presided by the U.S. District Judge Freda L. Wolfson and U.S. Magistrate Judge Lois H Goodman.
States Might Opt For Smaller Opioid Deal Amid Crisis

A Morgan Stanley analysis states that companies alleged the opioid crisis might make a smaller settlement with U.S. states in return for faster payments as local governments are facing a financial crisis due to the global pandemic outbreak.
According to analyst Ricky Goldwasser, a settlement amount of $18 billion with a $14.6 billion upfront payment would be considered sound for the three-drug distributors AmerisourceBergen Corp., McKesson Corp., and Cardinal Health Inc.
The novel coronavirus has taken the focus from the opioid lawsuits claiming drugmakers and distributors of intentionally not informing about the addictive nature and consequences of the drug and failing to halt large suspect purchases.
Last month, the U.S. Attorney for Colorado, Jason R. Dunn, announced that Omnicare Inc., a subsidiary of CVS Health, has agreed to compensate $15.3 million in penalties to settle allegations of dispensing opioids and other drugs without a valid prescription.
All opioid lawsuits are consolidated under MDL No. 2804, presided by U.S. District Judge of the United States District Court for the Northern District of Ohio Dan Aaron Polster.
The term “opioids” consists of compounds that are extracted from the poppy seed as well as semi-synthetic and synthetic compounds with similar properties that can interact with opioid receptors in the brain. Opioids are normally used for the treatment of pain and include medicines such as morphine, fentanyl, and tramadol.
Their prolonged use, misuse, and use without medical supervision can lead to opioid dependence and other health problems. The pharmacological effects of opioids can cause breathing problems, and overdose can lead to death.
Opioids are a class of drugs that act on the nerve cells in the body and brain to relieve pain. There are different types of opioids like Buprenorphine, Butorphanol, Codeine, Fentanyl, Oxycodone. Hydrocodone, Hydromorphone, and Hydromorphone are opioids that are not commonly used.
Opioids are on the market for ages and have been used basically for pain relief for post-surgical pain, cancer-related pain, chronic or persistent pain. Opioids when used in proper dosage and along with a combination of other pain treatments, work in relieving pain successfully, unless there is a misuse or abuse of the drug.
Companies manufacturing Opioids convinced the medical community that these medications were not addictive and were purely beneficial. This belief raised the number of prescriptions and sales unwarrantedly, resulting in a mass misuse of these drugs, to the extent that this was identified by the U.S. Food and Drug Administration (FDA) as a public issue and named it an 'Opioid crisis'.
According to WHO, about 0.5 million deaths are due to drug use. Almost 70% of these deaths are related to opioids, and more than 30% of those deaths are caused by overdose. Opioid overdoses have increased in recent years in many parts of the world. In the United States of America (USA) the number of people dying due to opioid overdose increased by 120% from 2010 and 2018, and two-thirds of opioid-related overdose deaths in 2018 in the USA involved synthetic opioids, including fentanyl and its analogs.
Long Term Users Of Elmiron Unaware Of Eye Damage Risks
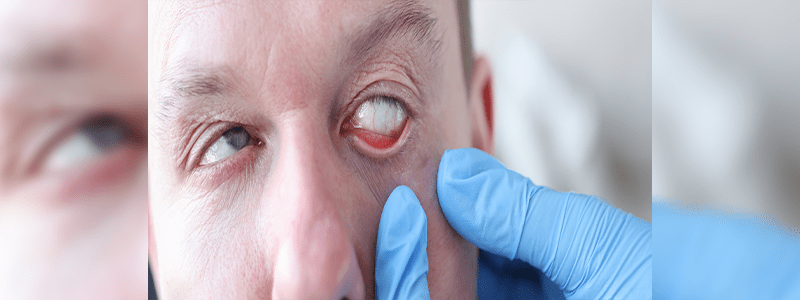
The growing number of Elmiron lawsuits indicate that several long term users are now learning that the interstitial cystitis drug may be the cause of vision loss and other similar symptoms they have been experiencing for years.
The drug has been on the market since the late 1990s, and it is the only drug approved to treat pain associated with diseases like interstitial cystitis, or IC - a condition that affects millions of people living in America every year. IC is more frequent in women, and many use Elmiron to treat the disease.
Thousands of people may have taken the drug and may have been exposed to the risk of potentially permanent or partial vision damage.
Prior to June 2020, Elmiron’s prescribing information provided by Janssen Pharmaceuticals listed the drug’s side effects and warnings but did not warn about the risks of maculopathy. A warning about retinal pigmentary damages is the current prescribing information.
Elmiron, also known by its generic name pentosan polysulfate sodium or PPS, is an oral prescription drug manufactured by Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson, and is used to treat pain/discomfort caused by bladder disorder interstitial cystitis or IC.
Starting in 2018, studies began to reveal irreversible vision problems associated with the long-term use of Elmiron.
The macula is the backside of the retina. The retina senses light and transmits a signal to the brain, allowing people to see. So retina damage affects a person’s vision and further leads to loss of vision. Elmiron may cause damage to a part of the retina called the macula, causing vision difficulty. The kind of maculopathy associated with Elmiron is called pigmentary maculopathy. According to studies, this type of maculopathy is unique to Elmiron users.
The damage may stop after taking medication if caught in its early stages. But in the late stages, the disease can lead to permanent blindness. Common visual symptoms stated in studies were difficulty reading and difficulty adapting to dim lighting.
The drug marketed in the U.S. does not contain a warning label about the eye damage risks, whereas the manufacturer of the Canadian version added the information about the side effects last year. The warnings were added after a series of independent studies, and case reports indicated that the users might face a rare form of eye damage known as pigmentary maculopathy.
Elmiron, also known by its generic name pentosan polysulfate sodium or PPS, is an oral prescription drug manufactured by Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson, and is used to treat pain/discomfort caused by bladder disorder interstitial cystitis or IC.
Starting in 2018, studies began to reveal irreversible vision problems associated with the long-term use of Elmiron.
The macula is the backside of the retina. The retina senses light and transmits a signal to the brain, allowing people to see. So retina damage affects a person’s vision and further leads to loss of vision. Elmiron may cause damage to a part of the retina called the macula, causing vision difficulty. The kind of maculopathy associated with Elmiron is called pigmentary maculopathy. According to studies, this type of maculopathy is unique to Elmiron users.
The damage may stop after taking medication if caught in its early stages. But in the late stages, the disease can lead to permanent blindness. Common visual symptoms stated in studies were difficulty reading and difficulty adapting to dim lighting.
The drug marketed in the U.S. does not contain a warning label about the eye damage risks, whereas the manufacturer of the Canadian version added the information about the side effects last year. The warnings were added after a series of independent studies, and case reports indicated that the users might face a rare form of eye damage known as pigmentary maculopathy.
Elmiron, also known by its generic name pentosan polysulfate sodium, or PPS, is an oral prescription drug that is used to treat pain/discomfort caused by bladder disorder interstitial cystitis, or IC.
The drug is manufactured by Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson, and was approved by the U.S. Food & Drug Administration (FDA) in 1996. The drug is approved under the Orphan Drug Act or ODA, which gives special status and incentives to sponsors, or manufacturers, of medications that treat rare diseases.
Interstitial cystitis, a chronic, progressive, and debilitating urinary bladder disease has affected more than 1 million people in the United States, primarily women.
The bladder wall's protective lining is made of glycosaminoglycans, or GAG layer, composed of a coating of mucus, which protects the bladder wall from bacteria and irritating substances in the urine. The lining is damaged in people with IC, due to which the irritants from the urine cause inflammation in the bladder wall.
Researchers are not sure how the drug works but believe that it works by forming a synthetic GAG layer on the bladder wall and protecting it from harmful/irritating substances present in the urine.
Several studies and a growing number of lawsuits filed in recent years claimed that long-term exposure to Elmiron has serious eye-related side effects and causes maculopathy, an eye disorder affecting the macula, central part of the retina, which is a major cause of blindness. Many experts have referred to the retinal disease as “Elmiron maculopathy” or “PPS maculopathy.” It is also a weak blood thinner and, therefore, may increase the risk of bruising/bleeding.
Medical practitioners in the U.S. recently started learning about the link through medical literature, but the warning label in the state still contains no mention of maculopathy or vision problems.
Due to the failure to disclose the safety warning, the number of lawsuits being filed throughout the U.S. is growing day by day, leading to a shocking reaction from long-term users who have been dealing with vision problems and were unaware of the link.

